Introduction
Proteins are complex organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and usually Sulphur or phosphorus.They are composed of one or more chains of amino acids.
Proteins are fundamental components of all living cells and include many substances, such as enzymes, hormones, and antibodies that are necessary for the proper functioning of an organism.
They are essential in the diet of animals for the growth and repair of tissue and can be obtained from foods such as meat, fish, eggs, milk, and legumes.
Basic Structure of a Protein
Proteins are made up of hundreds or thousands of smaller units called amino acids, which are attached to one another in long chains.There are 20 different types of amino acids that can be combined to make a protein. 9 are essential and 11 are non essential.
The sequence of amino acids determines each protein’s unique 3-dimensional structure and its specific function.
The bond which unites the two amino acids is called a peptide bond.
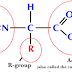
One amino acid has
1 carboxyl group (COOH)
1 amino group or nitrogen group (NH2)
1 hydrogen (H)
And 1 functional group (R)
NH2CHRCOOH.
Classification of protein based on origin
Classification of protein based on characterization
Classification of protein based on function
Structure of Protein
Effect of heat on proteins (Denaturation)
At the molecular level, natural proteins are shaped like coils or springs. When natural proteins are exposed to heat, salt, or acid, they denature—that is, their coils unwind.
When proteins denature, they tend to bond together, or coagulate, and form solid lumps.Coagulation is what which occurs on the physical structure during denaturation. As the proteins gets denaturated it become a hard or solid structure which is called as coagulation.
An example of this is a cooked egg white, which changes from a transparent fluid to an opaque solid. As proteins coagulate, they lose some of their capacity to hold water, which is why protein-rich foods give off moisture as they cook, even if they are steamed or poached. Denatured proteins are easier to digest than native proteins.
Factors affecting denaturation are
Agents such as acids, alkalis, saltsIncrease in temp
Extensive beating
Effect of heat on proteins (Denaturation)

Effect of heat on proteins (Denaturation)

Stages in heat denaturation
Unfolding of helix of the protein molecules as the cross wise link holding helix is disturbed.R groups are exposed. Re-bonding takes place between adjacent R groups of protein molecules leading to aggregation of the molecule bringing about increased viscosity. This is the first change in denaturation which is called as surface denaturation.
When sufficient proteins have united, the protein molecules are no longer dispersed as a sol. At this stage protein is said to have coagulated (second stage) i.e. water is held in the capillary spaces formed by the united proteins molecules and the coagulation of protein forms a gel.
If the liquid is separated from the coagulated protein, the protein is said to be precipitated or flocculated i.e. curdling take place (third stage of denaturation)
Effects of Denaturation
Properties of denatured proteins are completely different from their native form.Denatured proteins are easily attacked by proteolytic enzymes, e.g. cooked meat is more easily digested than raw meat.
They show decreased solubility, e.g. cooked egg white is not soluble in water.
They loss their biological activity as enzymes are destroyed e.g. browning does not take place in boiled potato.
Denatured proteins lose their ability to crystallize.
There is an increase in viscosity of food.
Heat Denaturation results in improved flavour and texture e.g. cooking improves flavour in meat and eggs give structure and improves texture of cakes.
Denaturation of food is irreversible unless it occurs under very mild conditions.
Factors affecting Denaturation
pH – Denaturation is brought about by controlling pH .Heat – when egg white is heated at 600 C the protein ovalbumin gets denatured. As temperature increases, coagulation takes place and egg white separates out as a solid.
Surface Denaturation – this is brought about by mechanical means e.g. beating egg white or milk to a foam. If the foam is heated, as in egg white foam, it becomes firm due to the coagulation of ovalbumin.
Salts – when present in a high concentration, it precipitates proteins out of solution and disperses them e.g. cured ham baked in white sauce.
Moisture – low moisture levels cause less Denaturation than higher moisture levels at the same temperature.
Functional properties of proteins
Gelation
Gelatin is partially degraded protein prepared from collagen.Collagen is the intercellular cementing substance between cells.
Skin, ligaments and bones are hydrolyzed by dilute acid or alkali, breaking collagen molecules into shorter fibrous molecules called gelatin.
Dry gelatin is soaked in cold water for preliminary hydration before adding some hot water. The mixture is stirred to form a sol.
Alternatively, hydrated gelatin is heated in a double boiler for gelatin to dissolve. The concentration gelatin sol is added to gel a liquid stirring thoroughly to prevent from solidifying into rubbery strands or lump.
Foamability
Egg white is a viscous sol with proteins dispersed in it. It can be beaten into a foam.The protein ovomucin, ovogloulin and conalbumin are necessary to form a fine foam with small air cell. As air is incorporated into the liquid, protein molecule collect at the air-water interface. When more air is incorporated, the water layer gets thinner and protein molecule gets stretched and unwinds from their coiled structure.
Surface Denaturation takes place and makes the foam rigid and when heat is applied protein coagulate forming a permanent foam.
Emulsification
It is the most important process in the manufacturing of many formulated foods. Emulsion represents a heterogeneous mixture of fat globules. Food emulsions can be of the oil in water (O/W) or water in oil (W/O) type. The difference between O/W and W/O emulsions is that an O/W emulsion commonly exhibits a creamy texture, while a W/O system has greasy textural properties.Protein emulsifying activity is the ability of the protein to participate in emulsion formation and to stabilize the newly created emulsion. The emulsifying capacity is the ability of the protein solution or suspension to emulsify oil. Emulsifying properties are useful functional characteristics which play an important role in the development of new sources of plant protein products for uses as foods. Proteins are the components that dominate in most food emulsions.
Viscosity
The viscosity of a solution is related to its resistance to flow under an applied force.Viscosity or consistency of the products is very important for the consumer acceptance of several liquid and semisolid-type foods (e.g. soups, beverages). High-molecular-weight polymers such as proteins greatly increase viscosity.
The viscosity behavior of proteins is affected by several variables including size, shape, protein-solvent interactions, hydrodynamic volume and flexibility in the hydrated state.
COMMERCIAL USES OF PROTEINS
The major role of protein in food preparation includes the ability of proteins toForm foams
Bind water and form viscous sols and gels
Get coagulated by heat
Exhibit emulsifying properties
Show enzymatic activity
Egg, milk and gelatin are used to make gels, foams, whips, soufflés, meringues, custard, cakes, puddings, confections, soups and sauces.
Protein extract from natural and novel sources are being extensively used in the manufacture of convenience food for catering systems and for low-cost feeding programmes which rely on protein to bridge the protein calorie gap.
COMMERCIAL USES OF PROTEINS
Other commercial uses of protein
TEXTURED VEGETABLE PROTEIN (TVP)
Plant protein can be used to produce textured protein products also called protein analogs. They form an important substitute for expensive animal products. Textured vegetable protein (TVP) includes proteins manufactured from soya bean, ground nuts and other oilseed after oil has been expelled. Proteins can also be extracted from green leaves and certain grass and species of microorganisms such as yeast, mould and bacteria.
TEXTURED VEGETABLE PROTEIN (TVP)
The advantage of using TVP is as follows:- They can be used as substitute for real meat in curries, biryanis etc.
- Alternatively they can be used along with real meat as meat extender by mixing it with meat products as in cutlets, keema matar etc.
- They are equally nutritious as meat and cheaply priced thereby cutting down the costs
- They are widely used in the food processing industry.
- They are acceptable to vegetarians and are used in nutrition feeding programmes.








No comments:
Post a Comment